- Ismael Ávila
- no comments
Introduction: Understanding DTx
We have heard and read in many sites that Digital health is becoming more present in the health field. But, this term includes many others like Digital Therapeutics (DTx), that is a subset of Digital Health, the topic about what this article talks about. Here you will find a study on the state of the art of existing solutions, classification, indications, regulatory, challenges and opportunities and more.
DTx is here to stay, and many institutions like hospitals and pharmaceutical companies are increasing their interest for DTx, where there is a special use in chronic and behavioral disorders. But, nowadays it is tough to separate unproven or low-value applications from those with proven therapeutic value. Evidence on safety and efficacy are difficult to obtain what represent big barriers to access the market, but many opportunities are just around the corner.
But, before entering in the report, it is important to first clarify the differences between some topics that could create confusion, because sometimes they sound very similar and are used interchangeably, let’s see the differences between Digital health, Digital medicine and Digital Therapeutics. Once understood this easy or difficult differences we could dive in the DTx world that is such an adventure and a land to discover.
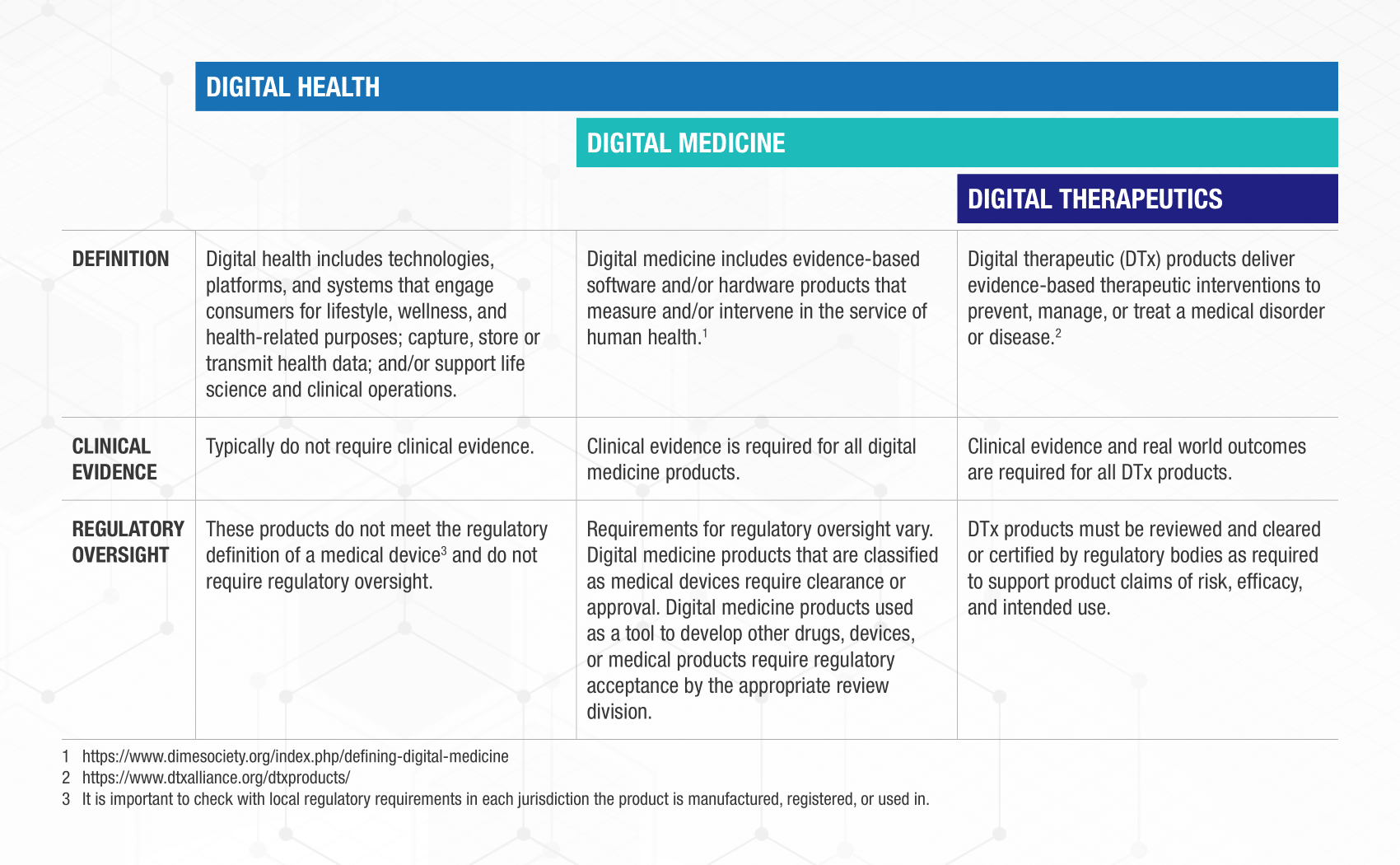
Digital therapeutics are not only disruptive to the healthcare system through facilitating remote patient management and personalized treatment, they also have the potential to impact on healthcare cost, regulatory processes, and the way pharmaceutical companies will do businesses and clinical trials. In the following diagram is shown how Digital Tx are prescribed:
DTx subclassification
During the search on internet we have found two ways to classificate the existing DTx solutions:
- Based on its target product profile
DTx can be distinguished based on the primary function. Each DTx treatment belongs to one of the following categories:
| Address a medical condition | Manage or prevent a medical disorder or disease | Optimize medication | Treat a medical disease or disorder |

Source: link |
Source: link |

Source: link |

Source: link |
- Based on how they complement other treatments
Another way to classificate Digital Tx is regarding how they can be used:
| Standalone | Augment | Complementary |
Source: link |
Source: link |
Source: link |
| Standalone DTx can work individually or even replace pharmacological interventions and they can be used at the same time with other treatments. | Augment DTx are made to work at the same time with pharmacological interventions to augment existing therapies. | Complement DTx improving selfmanagement of the condition and related healthcare factors as they work in combination with drugs. Complement with medications, devices, and other therapies to engage patients and improve the overall quality, cohesion, outcomes, and value of how care is delivered. |
DTx is a novel category of therapeutic options. In addition to existing pharmacological, non-pharmacological (surgical, radiation, and physical), and psycho-behavioral therapy, it can cover therapeutic areas that have previously not been well described. However, the current stages of most DTx-based products correspond to the convenient replacement of already existing treatment settings, such as that for cognitive behavioral therapy (CBT) and reminiscence therapy. The conceptual position of DTx in relation to the existing therapeutic options is represented in the following figure. (5)
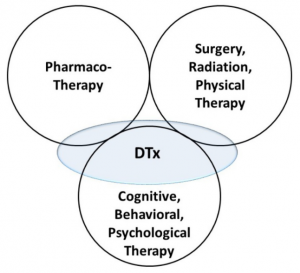
What is and what is not DTx
Another interesting way to analyze DTx is trying to understand what is and what is not a DTx solution, evaluating different elements.
| YES | NO | |
| Functioning | Relies on algorithms for its mechanism of action as well as the algorithmic information management. | A tool that not prevents, manages, or treats medical disorders or diseases. |
| Indications |
Focused on one condition. It can be used independently or in combination with other treatment modalities. | It is focused on various conditions. |
| Regulatory |
It needs to assure that it is safe and proven therapeutic value by evidence. | Unsubstantiated claims about clinical benefits |
| Way to access to the market | B2B2C | B2C |
| Performance indicators | Efficacy and safety | Number of users, usage, direct turnover |
| Reimbursement | It can be reimbursed by payer | It can be subscribed by consumers. |
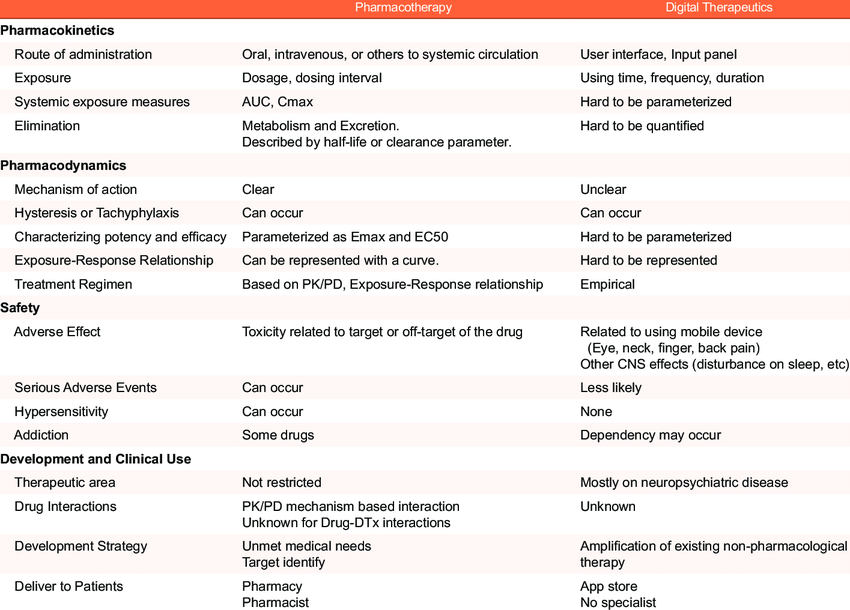
Comparing Digital Tx vs. Pharmacotherapy
The similarity between pharmacotherapy and DTx is that they are both medical treatment methods.
However, the fundamental difference is that the therapeutic effects of DTx are present in the absence of an active substance in the systemic circulation. Therefore, all pharmacokinetic (PK)/pharmacodynamic (PD) parameters related to plasma concentrations cannot be derived and used for response optimization. However, similar exposure concepts not based on concentrations can be applied. The dosage, frequency, time (total amount of used time and/or completed percentage out of required sessions), or modules can be all regarded as the exposure of DTx.
The comparisons between pharmacotherapy and DTx from the point of view of clinical pharmacology are summarized in the following table. In this table, there are several fundamental characteristics of DTx that do not apply to conventional pharmacology. However, there is an opportunity to apply pharmacology concepts by quantitative modeling (pharmacometrics), because the input and response variables of DTx are all digitally implemented and quantifiable. (11)
Exposure-response relationship of digital therapeutics
Exposure-response relationship is a core principle of clinical pharmacology for developing optimal drug therapy. However, there are few dose-response studies related to DTx. In the near future, the use of pharmacometrics for the exploration of dose-response relationships, and real-world big data analysis will spread widely because DTx can generate large data that can be used for pharmacometrics analysis.
Exposure-response relationship of digital therapeutics
Exposure-response relationship is a core principle of clinical pharmacology for developing optimal drug therapy. However, there are few dose-response studies related to DTx. In the near future, the use of pharmacometrics for the exploration of dose-response relationships, and real-world big data analysis will spread widely because DTx can generate large data that can be used for pharmacometrics analysis.
Market landscape of DTx
The value of the global DTx market is estimated at USD 1.8 billion in 2018, which is expected to reach USD 7.1 billion by 2025. A recent report estimates the biggest applications for DTx to be diabetes and weight loss shortly, with other applications likely to be observed in conditions such as chronic obstructive pulmonary disorder (COPD), developmental disorders (with the use of computer games), and post-traumatic stress disorder.
The arrival of DTx is reforming the landscape for new medicines, product reimbursement, and regulatory surveillance. As a result, new data-sharing processes and payment models will be formed soon to incorporate these products into the broader treatment plans and regulatory structure for drug and device approvals. Recently, there has been a massive investment of USD 12.5 billion in digital health ventures. (7)
When we define the market landscape, it is always useful to analyze the stakeholders involved. In the following graphic, there are identified the main stakeholders which who the DTx companies will need to interact or offer a value proposal along their development and implementation plan in order to arrive patients:
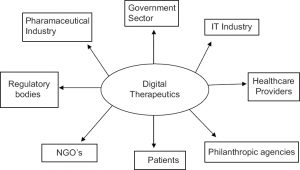
Source: 2019 DOI: 10.12793/tcp.2019.27.1.6. Jae-Yong Chung. Digital therapeutics and clinical pharmacology
Market drivers in Digital Tx
Appart from the stakeholders in the market landscape, several elements are origining that Digital Tx will become more present in the upcoming future. This is possible due the following market drivers (9):

Growing population of technology users
The increasing internet penetration and growing adoption of smartphones are increasing patient engagement, reducing patient burden, and delivering novel outcomes.
Increased emphasis on cost-effective healthcare
The DTx market grew due to industry needs for cost-effectiveness, safety, and increasing emphasis on preventive healthcare.
Rising aging population
The burden of diseases has moved from tackling infectious diseases to managing long-term conditions such as diabetes, CVD, and cancer.

Significant investments in DTx
Pharmaceutical companies, medical technology companies, and payers will invest more in DTx as their adoption will see significant growth.
Increased focus on value-based healthcare models
The global health sector is becoming more focused on patient outcomes, and payment for value not volume.

Focus on prevention and precision medicine
DTx brings precision to therapy and the ability to personalize treatment, giving them an important role to play in the transition from the traditional fee-for-service to value-based care.
Intellectual Protection Strategies
In the rapidly developing, highly competitive digital health industry, you can thing that the use of patents could be the “option”, but in this DTx industry there are two relevant elements to strategically protect the solution:
- Brand protection
Brand protection will confer to the project a special, recognziable and differentiator aspect to ease the market access. For that reason a strong brand protection will also offer the opportunity for a great market entry.
- Trade secrets to protect technologies
Trade secrets may protect frequent incremental technological improvements in digital technologies, such as software updates, which may not be regarded as novel or inventive under patent law. Further, as lead time is often a major competitive advantage in the digital health industry, trade secrets can be valuable for pioneering companies to protect their market positions. In addition, software, algorithms, and databases underlying digital applications may all be kept secret and fully protected by encryption to prevent reverse engineering by the public after sale.
Market access strategies
Good market access strategy consists of understanding the market access environment, for that reason is important to:
- Define standards of safety and efficacy for the healthcare systems and regulatory requirements
- Design clinical trials, with essential efficacy and safety evidence (such as cost-effectiveness)
- Collaborate among DTx stakeholders: companies and payers, providers, and pharmaceutical companies
- Identify the unmet needs that a DTx fulfills, and pricing the therapy accordingly
- Analyze the current treatment options and the future competitive landscape
Some DTx will have the opportunity to be reimbursed through insurance plans in the same way as drugs or medical technologies.
Reimbursement
Some DTxs can be reimbursed through insurance depending on the medical benefit they offer, like the case of WellDoc’s Blue Star and Pear Therapeutics’ reSET. DTx manufactures can give licenses to other interested manufacturers and also there is opportunity of selling data to the same therapeutic field manufacturers for their future development of products.
There are several reimbursement considerations for DTx to take into account:
- Reimbursement is crucial for DTx to become more adopted in the healthcare system and high in the priorities of payers.
- Some DTx will have the opportunity to be reimbursed through insurance plans in the same way as drugs or medical technologies.
- Current reimbursement models are not suited for all DTx; alternative payment methods, such as an outcome-based payment model, are likely to be more successful and effective for DTx.
Anyway, there is a widespread uncertainty from healthcare authorities and payers about the value of DTx, due to the nascent state of the market. In many countries, reimbursement is difficult to navigate, often with lengthy processes and unclear requirements. Reimbursement and payment for products are still not widely adopted in clinical practice and this limits DTx market access.

Link: https://research2guidance.com/where-is-the-money-in-digital-health-the-roadmap-to-digital-health-app-reimbursement-in-europe/
Platforms to follow the last advances and news
The main reference institution in the Digital Tx arena is the Digital Health Alliance: https://dtxalliance.org/join-dta/.
Deals in the Digital Tx arena
Some of the examples of real deals that happened between DTx and other companies are represented in the following table:

The market is evolving through six main therapeutic areas. In the following table is shown the maturity level of the solutions in different therapeutic areas taking into account the different applications of Digital Tx. (12)
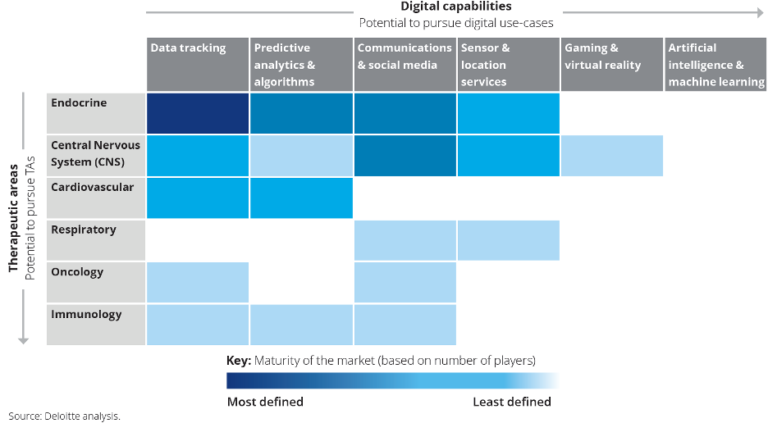
Solutions landscape in medical areas
Several companies found on internet and in the literature have been highlighted. In order to overview the different existing solutions, they have been classified by therapeutic area/indication:
| Company | Description |
 |
Metabolism and endocrinology/Diabetes
Blue Star was the first digital phone app approved by the FDA to manage diabetes. BlueStar, a DTx developed by WellDoc, provides a 24/7 platform for delivering real-time education and coaching to guide T2D patients in the self-management of their disease in improving glucose control, by using real-time data and feedback to support healthy behaviors in optimal treatment decisions or pathways. |
| Metabolism and endocrinology/Diabetes
It is a lifestyle modification program for diabetes prevention clinically proven to be more efficacious than lifestyle modifications launched by Omada Health, Canary Health, and Blue Mesa Health. It includes close monitoring of patients in terms of diet, weight, and physical activity and associates them with health coaches, clinicians, or peer groups. This helps to intervene at the time of exacerbations and reduce the frequency of symptoms. |
|
 |
CNS/Schizophrenia
Thrive digital app is used along with antipsychotic medication and targets positive symptoms of schizophrenia. |
 |
CNS/Stress incontinence
INNOVO is the first transcutaneous electrical stimulator in women to treat stress urinary incontinence. It offers a safe, clinically effective, and noninvasive choice. |
 |
Neurology/ADHD
Endaavor is an adaptive sensory estimulus software delivered via video games for ADHD, autism, major depressive disorder, multiple sclerosis and inflamatory disesases. First, the U.S. Food and Drug Administration has approved this video game called EndeavorRx as a treatment for children with attention-deficit/hyperactivity disorder. |
 |
Neurology
2Morrow is a digital health company focused on delivering wellness and behaviour change programs via smartphone apps for aiding healthcare issues including smoking, vaping, weight, stress/anxiety, and chronic pain. In Oct 2019, 2Morrow in collaboration with Washington State Department of Health developed and launched its first digital health app for quitting vaping in young and adults. |
 |
Neurology
Propietary cognitive and neurobehavioral platform that optimizes user engagement for a variety of indications, including Major depressive disorder (MDD), migraine, insomina and acute coronary syndrome. |
| Neurology
Big Health is an utomated and personalized cognitive behaviroal programs for mental health, including insomnia and anxiety. |
|
 |
Neurology/Suicide Prevention App
Besafe is an application for preventing suicide of children or adolescents. As teenagers use smartphones and applications, Besafe app is an effective way to prevent teenagers who are thinking about suicide. The application prevents suicide by transmitting psychology education and offering crisis coping tools, to prevent deliberate self-harm. |
 |
Neurology
MoodHacker is accessible through a website and mobile application that combines the skills and techniques of positive psychology and cognitive treatment for depression, which is a funding tool for self-management of depression based on Cognitive Behavioral Therapy (CBT). The goal is to motivate employees to work, improve their mood, reduce depressive symptoms, and prevent escalation into clinical depression. |
 |
Neurology/Autism
It provides a clinically validated assessment to screen for children’s risk of developmental delay and autism. It can be used to expedite a diagnosis from clinicians. It also includes personalized activities parents can do with their child, based on their unique strengths and areas for improvement. |
| Respiratory/Asthma
The app myCOPD gives patients 24/7 access to a platform to educate, monitor symptoms, optimize self-management, and improve both their adherence and quality of inhaler administration. The myCOPD self-management plan helps patients understand what medication to take and when. |
|
 |
Respiratory/Asthma
The free application allows users to easily and quickly log their asthma activity, their medications, causes of their asthma in the form of a diary. Users may share the diary and a color graph chart of their asthma activities with their physicians to be included in their medical records. It combines peak expiratory flow (how fast a person can breathe out air) and symptom data chart. Its main significance is to make it convenient for patients to record their peak flow and data, and take appropriate measures as directed by the doctor. At the same time, researchers can track these data and use it to determine the cause and region of asthma flare formation. |
 |
Sleep/Insomnia
An online therapy program with visual exercises to induce sleep. It claims to replace sleeping pills, being more efficacious and cost-effective. |
 |
Pharmacy/Curb substance abuse disorder
ReSET is used in conjunction with the standard treatment. It claims to improve clinical outcomes by increasing patient’s adherence to treatment. |
 |
Pharma Patient Engagement
reSET is a DTx intended to provide Cognitive behavioral therapy (CBT), as an adjunct to a contingency management system to treat Substance Use Disorder (SUD). The app provides CBT through mobile and web-based applications, monitors patient data in real time, and detects behavioral and biological changes in condition regularly. |
 |
Traumathology & Orthopedics
Uses neurologic music therapy via a combination of a snesors, music, and AI to measure and improve walking. |
 |
Oftalmology
Cognitive visual function assessment and training of visual dysfunctions providing a personalized plan for each patient and specialist |
Regulatory in DTx
The regulatory process works different depending on the countries (information and pictures soruce: 9):
| Region | How does it work there? |
 |
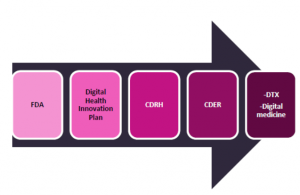
In its Digital Health Innovation plan, the FDA is piloting a novel risk-based approach to regulating Software as a Medical Device (SaMD) called the Precertification Program, which would enable companies that can demonstrate specific requirements to speed up the approval process. This will reduce the time to market. |
 |
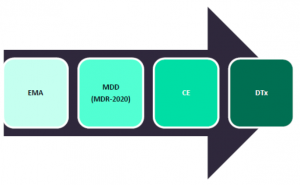
The new Medical Devices Regulation 2017/745 (MDR) replaces the former MDD. The MDR introduces new definitions, classifications, procedural requirement, and rules for medical devices and also intensify and speed up the process of approval. There will be more transparency of information on the benefits for patients, residual risks, and a thorough assessment of the overall risk/benefit. Importantly, the new MDR will reclassify some digital technologies from the lowestrisk Class I to Class II+ devices. This reclassification will increase also the standard of evidence required to receive a CE mark, which is a requirement for use and reimbursement in Europe, and will increase post-marketing surveillance expectations from these manufacturers. |
 |
DTx are not as well-regulated as pharmaceutical products and several challenges are present when it comes to market access and reimbursement, especially for DTx, which are not supported by enough clinical evidence. NICE is still behind compared to the US regulations and FDA guidance and legislation around digital health appraisal and requirements for real-world evidence. Currently NICE, in partnership with NHS, Public Health England, MedCity, and DigitalHealth.London, developed an evidence standards framework for digital health technologies to ensure new technologies are clinically effective and offer economic value. |
 |
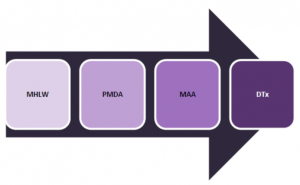
Japan’s regulatory agency, the Ministry of Health, Labour and Welfare (MHLW) provides marketing authorization and implementing safety standards for medical devices and drugs. In collaboration with the MHLW, the Pharmaceutical and Medical Device Agency (PMDA), an independent agency that is responsible for reviewing drug and medical device applications, assesses new product safety, develops regulations, and monitors postmarket safety. DTx are generally reviewed under Marketing Authorization Applications (MAA) and by external advisory experts. |
 |
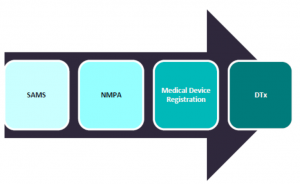
In China, the National Medical Products Administration (NMPA) regulates medical devices, including DTx, within its Department of Medical Device Registration that is responsible for pre-market approvals. The NMPA is a ministry-level organization that is overseen by the State Administration for Market Supervision (SAMS). Products are also reviewed by Market Authorization Holder (MAH) as they must ensure the quality and meet all applicable requirements, submit self-inspection reports to authorities every year, and maintain their products. |
TOP 5 Digital Tx Challenges
- Clinical evidence for efficacy and safety is still sparse
Payers would prefer to offer reimbursement for digital therapy upon achieving better clinical outcomes within a time frame. Now, it is tough to separate unproven or low-value applications from those with proven therapeutic value. - Cibersecurity
The cibersecurity of patient’s data is a big responsibility for the DTx companies. - Dose exposure and response
The investigation of dose-response relationships and their application to the designing of optimum therapies is a desirable future direction for DTx. Pharmacological concepts need to be applied to the development and optimization of treatment regimens. - The remote informed consent and who is receiving the treatment
In mobile device based clinical research, the remote informed consent process raises privacy concerns. Hitting the “OK” button by a subject cannot be considered sufficient consent. - No fast regulatory tracks
Implement fastest regulatory tracks for the preparedness of the documentation in order to pass the regulatory approval.
TOP 5 Digital Tx Opportunities and advantages
Appart from the challenges that Digital Tx solutions need to overpass, there are also opportunities that due the nature of these solutions can be very present:
- Direct access to patients
In Practice of Primary Care, DTx offers the physicians an option to provide treatment anywhere and anytime, transcending the physical borders of a clinic or a hospital. - Personalization of the therapy
The continuous monitoring of patient vitals, can enable primary care physicians to monitor and track response to the prescribed therapies, thereby aiding to provide personalized treatment, for example in conditions such as depression, anxiety, chronic diseases such as diabetes, cardiovascular conditions such as hypertension, and pulmonary diseases like Chronic obstructive pulmonary disease (COPD). The life cycle of the DTx can be updated rapidly through regular software updates. - The VR technology
VR in DTx applies simulation to enable people to learn by experiencing real-world situations. For example, through VR, patients go into situations and learn how to think, feel, and act differently.
- DTx is generally considered safe
The DTx solutions don’t have to produce toxicity or other associated side effects. - Costs optimization
Minimizes costs and time associated with attending a hospital or doctor’s clinic and administrative tasks and routine communication, that could be focused on more time spent on treating patients.
Tips to evaluate utility Digital Tx solutions
DTx are required to have certain standards to be able to integrate into the healthcare systems. Let’s try to do a Health Technology Assessment and how to differenciate from useful to not useful solutions.
| Clinical Evidence-Based Therapeutic Benefit |
|
| Regulatory Approved and Data-Driven
|
|
| Accessibility Prescribed and Reimbursed
|
|
Digital Tx and Clinical Trials
DTx clinical trials are not as linear as drug development and given the nature of software, they need to be agile as time is the key to be successful clinically and commercially. The FDA has recognized this need for a new regulatory approach to evaluate rapidly and provide clearance for DTx to be used in clinical settings. Some of the key features of Digital Tx in clinical trials are:
- DTx trials allow collection of more clinical data, as data can also be collected passively at home compared to site-clinical trials.
- Virtual trials can cost half as much per participant compared to a site-based trial.
- They can speed up recruitment rates by making it more convenient to participate in trials.
- DTx could make clinical trials more patient-focused and also increase the opportunities for patient engagement.
The BioRegion has seen significant growth in companies that fall into this subsector over the past ten years, as well as the amount invested here, as can be seen in the 2020 BioRegion Report presented in September. While in 2010 there were only about 20 companies, 10 years later there are over 200: or a tenfold increase in number of companies. Investment in these companies has tripled in recent years, from €5.1 million in 2016 to €18.8 million in 2019, according to the report.

This growth reveals the subsector’s youth, which is 90% startups and has a joint turnover of €118 million. So, the average company is young, with under five years in the business, and no more than 10 employees. One important figure: 21% of these firms were founded and are led by women.
The Report classifies the DTx solutions in three typologies: Self-Management, Active Monitoring and Treatment. Doing a Zoom in the Treatment DTx companies there are 7:
 |
Virtual Bodyworks specialises in immersive virtual reality with applications in medical and social rehabilitation. |
 |
It is a Virtual Reality (VR) solution tailored for hospital usage to enhance patient’s experience during medical procedures. Their mission is to decrease patient’s anxiety levels and pain perception with a user-friendly and innovative system. |
 |
The Psious platform has more than 70 virtual environments and scenes specially designed to easily work on dozens of pathologies. The therapist can use the tool for multiple techniques (psychoeducation, gradual exposure, systematic desensitization, relaxation, distraction, acceptance and commitment, mindfulness, EMDR …) to work with their patients.
It supports the professional in their care for all kinds of disorders such as anxiety (phobias, panic, agoraphobia, generalized anxiety, public speaking, exams, etc.), attention management, eating disorders, OCD, ADHD, and pain management, among others. |
 |
Neuropsychological evaluation composed by a set of methods and techniques that allow to identify, describe and quantify cognitive, behavioral and emotional alterations. This platform allows us to offer effective and efficient personalized treatments for people who have suffered a neurological disorder. Although their fields of action cover a wide spectrum, they could be considered among the most common: stroke, dementia, Alzheimer, Parkinson, multiple sclerosis, schizophrenia, bipolar disorder, intellectual disability, etc. |
 |
Limbix is an investigational digital therapeutic solution for adolescent depression. If Limbix Spark is FDA cleared it will be the first prescription digital therapeutic designed to support adolescents with depression. Spark is a multi-week cognitive behavioral therapy based program focused on the completion of value-based activities that spark feelings of pleasure or mastery. |
 |
WIVI offers a complete cognitive visual function assessment and training of visual dysfunctions providing a personalized plan for each patient and specialist, with freedom of movement, high accuracy, real time measurement at an affordable price using 3D technologies. WIVI is a new immersive and interactive product based on 3D visualization and interaction which allows a comfortable and free way to assess the skills and capabilities of the visual system as well as the design and implementation of treatment by appropriate vision training for improvement and standarization. Various elements where images and 3D models are presented simulating different realistic scenarios with different visual challenges. |
 |
WeAR Technologies is a spin-off of the Institute of Research in Artificial Intelligence (IIIA CSIC), specialized in the monitoring of patients with mental and physical health problems. The device through the combination of artificial intelligence systems and weareble sensors, monitors the therapies that medical centers apply to patients with chronic or persistent health problems. The system optimizes the work of therapists and allows greater control and personalization of the therapies that apply to each patient. |
Appart of these companies showed above, there is a product from the Parc Taulí Research and Innovation Institute (I3PT) that is in via to be commercialized in order to reach society, either through exploitation and / or distribution licenses, or technology transfer agreements is EPPICS, from English Early prevention of the Post-Intensive Care Syndrome, aims to develop an innovative technological tool for the prevention of cognitive and emotional disorders associated with PICS. EPPICS is an innovative digital tool based on our previous proof of concept, which will improve patient recovery and comfort during the ICU stay. EPPICS has various exercises for the prevention of functional alterations at a cognitive and emotional level, and is integrated into a communicator for those patients who do not have the ability to communicate.
Great quotes
- “If we look at major trends, people are living longer, on average, so there’s a rise in chronic diseases associated with aging. Meanwhile, healthcare workers are in short supply, and physicians and nurses do not have enough time for patients. Yet everyone has powerful computers in their pockets that give them access to technology, education, and information. Put all that together, and it’s the perfect moment for digital solutions to come to the market, change behavior, and enhance health outcomes at scale”. –Bozidar Jovicevic, Global head of Digital Therapeutics, Sanofi
- “DTx are not just a toy in a happy meal to sell more pills” — Anonymous
- “Reimbursement, commercial distribution and patient/provider education are the 3 biggest challenges”– P. Hames, Big Health
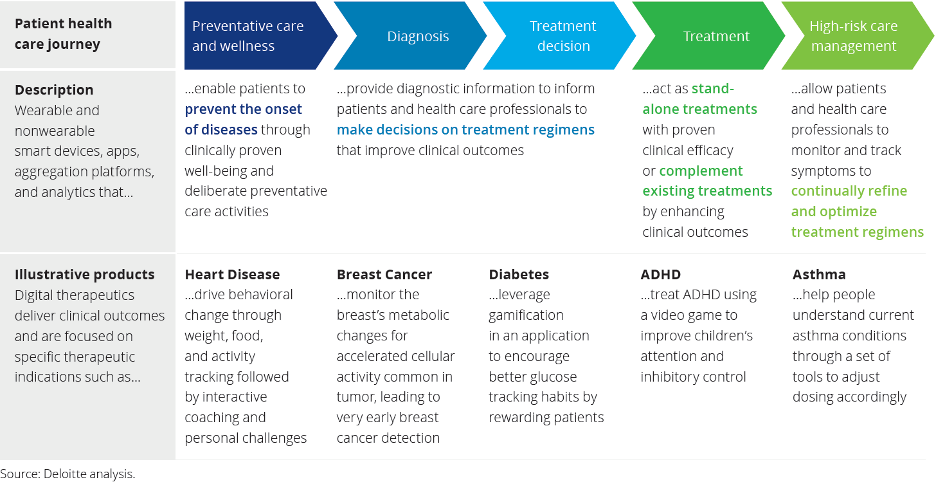
Patient health care journey
Also it is interesting to know how Digital Tx can be applied depending on the stage of the patient’s disease. In the following table is shown product examples in different therapeutic areas depending the disease’s stage:
Glossary
- E-patients
Patients who are “equipped, enabled, empowered, and engaged in their health and healthcare decisions.”[14] Today, the number of e-patients is on the rise. They are better engaged and want to participate in informed decision-making about their care. They expect informed answers for their medical and health technology-related questions from their physicians.[8] In such an aware world, the need for digital health and related tools becomes inevitable. - Hormesis
Is defined as a phenomenon in which a harmful substance gives stimulating and beneficial effects to living organisms when the quantity of the harmful substance is small (Sakai, 2006). - Software as medicine or behavioral medicine in a digital format
Therapeutics mimicking the product development path of pharmaceutical drugs which indicates online or mobile delivery of skills-based psychotherapy techniques. - Digital services
They modify patient behavior and help in driving a clinical outcome, for example, Omada health’s digital behavior platform for weight loss. - Adjunctive digital therapeutics
This tier complements traditional therapeutics, indirectly improving the clinical outcome, for example, Proteus Digital Health’s Discover medication.
- Digital drug replacement
They substitute conventional medicine. This requires stringent criteria in terms of clinical trials and the Food and Drug Administration (FDA) review, for example, reSET application for treating substance abuse disorder.
A host of companies are collaborating with health-care providers to shape up various DT programs in different diseases. Most of these initiatives are currently US based, but emerging trends from countries such as Japan, UK, and India are a positive development.
Articles and references
- https://www.bostonhealthcare.com/building-evidence-based-value-propositions-digital-therapeutics/
- 2020 DOI: 10.4103/jfmpc.jfmpc_105_20. Amit Dang, Dimple Arora and Pawan Rane Role of digital therapeutics and the changing future of healthcare
- DTA. Digital therapeutics alliance [homepage on the Internet] 2017. Available from: https://wwwdtxallianceorg/ Cited 2020 Jan 09.
- https://www.healthxl.com/blog/digital-health-digital-medicine-digital-therapeutics-dtx-whats-the-difference
- 2019 DOI: 10.12793/tcp.2019.27.1.6. Jae-Yong Chung. Digital therapeutics and clinical pharmacology
- 2020 DOI: 10.4103/jfmpc.jfmpc_105_20. Amit Dang, Dimple Arora and Pawan Rane Role of digital therapeutics and the changing future of healthcare.
- 2020 DOI: 10.4103/jfmpc.jfmpc_105_20. Amit Dang, Dimple Arora and Pawan Rane Role of digital therapeutics and the changing future of healthcare.
- 2020 DOI: 10.4103/picr.PICR_89_19. Raj Khirasaria, Vikramjit Singh and Angelika Batta. Exploring digital therapeutics: The next paradigm of modern health-care industry
- GlobalData_2019_Digital-therapeutics-and-their-impact-on-health-care
- 2020 DOI: 10.4103/jfmpc.jfmpc_105_20. Amit Dang, Dimple Arora and Pawan Rane Role of digital therapeutics and the changing future of healthcare.
- https://www.researchgate.net/figure/Conceptual-comparison-between-pharmacotherapy-and-digital-therapeutics-from-the-point-of_tbl2_332147628
- http://medicinman.net/2020/08/digital-therapeutics-new-frontiers-in-healthcare/
- https://www.americanbar.org/groups/intellectual_property_law/publications/landslide/2019-20/november-december/ip-strategies-red-hot-digital-health-industry/
- 2020 DOI: 10.4103/picr.PICR_89_19. Raj Khirasaria, Vikramjit Singh and Angelika Batta. Exploring digital therapeutics: The next paradigm of modern health-care industry
- https://www.bostonhealthcare.com/building-evidence-based-value-propositions-digital-therapeutics/
Ismael Ávila
Working in healthcare innovation and technology transfer, supporting biomedical projects from early research to clinical validation and market translation. My focus is on bridging science, care and entrepreneurship to create tangible impact in health.
All stories by: Ismael Ávila




Leave a Reply